The value of the observed and calculated molecular weight of silver nitrate is 92.64 and 170

The molar mass of ethylene glycol is 62.068 g/mol. B The number of moles of ethylene glycol present in 35.00 g can be calculated by dividing the mass (in grams) by the molar mass (in grams per mole): 35.00gethyleneglycol(1molethyleneglycol ( g)) 62.068gethyleneglycol) = 0.5639molethyleneglycol.
5.1g Finding molar mass from chemical formulae YouTube
Explanation of how to find the molar mass of AgNO3: Silver nitrate.A few things to consider when finding the molar mass for AgNO3:- make sure you have the co.
SOLVEDWhat mass of silver chloride can be prepared by the reaction of 100.0 mL of 0.20 M silver

Step2. Formula for molar mass. Molar mass = The atomic mass of element × number of atoms given in subscript. Step3. Molar mass of Silver nitrate. The formula for Silver nitrate is AgNO 3. Molar mass of AgNO 3 = The atomic mass of Ag+ The atomic mass of N+ 3× The atomic mass of O = 107. 87 g mol-1 +14.0067 g mol-1 + 3×16.00 g mol-1 = 169. 87.
SOLVED What is the molarity of a solution that contains 10.0 grams of silver nitrate that has
About Silver nitrate; Silver nitrate weighs 4.352 gram per cubic centimeter or 4 352 kilogram per cubic meter, i.e. density of silver nitrate is equal to 4 352 kg/m³; at 19°C (66.2°F or 292.15K) at standard atmospheric pressure.In Imperial or US customary measurement system, the density is equal to 271.6865 pound per cubic foot [lb/ft³], or 2.5156 ounce per cubic inch [oz/inch³] .
PPT Molar Mass PowerPoint Presentation, free download ID4296324

Find the molar masses of carbon (C), hydrogen (H), and oxygen (O). Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol.
The value of observed and calculated molecular weights of silver nitrate are 92.64 and 170
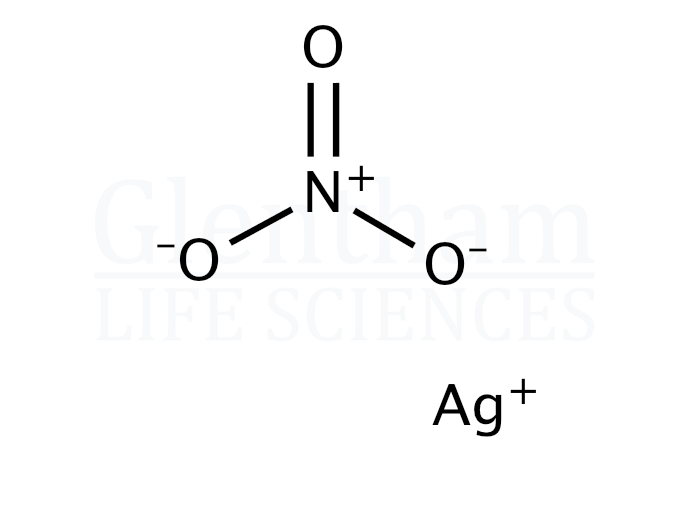
Silver nitrate. Formula: AgNO 3. Molecular weight: 169.8731. IUPAC Standard InChI: InChI=1S/Ag.NO3/c;2-1 (3)4/q+1;-1. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: SQGYOTSLMSWVJD-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet. Chemical structure: This structure is also available as a 2d Mol file or as a.
In the reaction of silver nitrate with sodium chlorid… SolvedLib

As mass/volume = molarity × molar mass, then mass / (volume × molar mass) = molarity. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 × 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. Simply type in the remaining values and watch it do all the.
PPT CHEMICAL QUANTITIES PowerPoint Presentation, free download ID586725
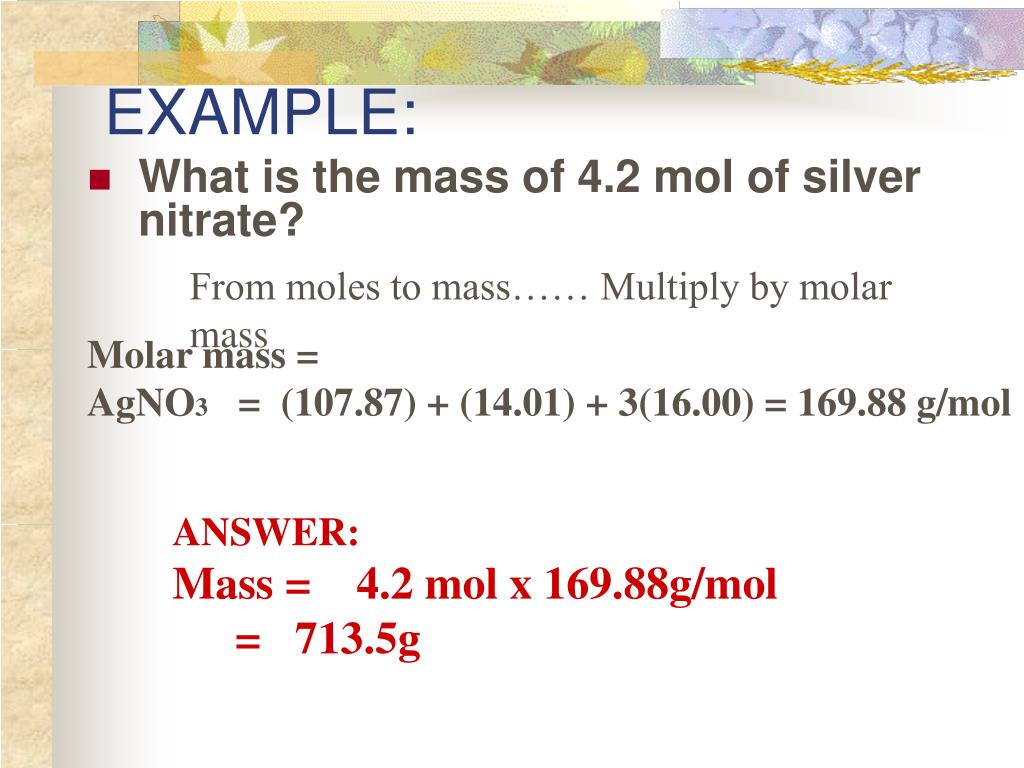
Silver Nitrate molecular weight. Molar mass of AgNO3 = 169.8731 g/mol. Convert grams Silver Nitrate to moles. or. moles Silver Nitrate to grams. Molecular weight calculation: 107.8682 + 14.0067 + 15.9994*3. Percent composition by element. Element: Silver Symbol: Ag Atomic Mass: 107.8682 # of Atoms: 1
Mass finder using molarity holysalo

Formula for silver nitrate = AgN O3. This gives the molar mass as. (107.87 +14.007 +(3 ⋅ 15.999)) = 169.874 grams/mole. 169.874 grams/mole The molar mass of a chemical is the molecular weight of the chemical in grams. i.e. 1 mole = (molecular mass) grams of the chemical. Molecular weight of silver nitrate = the sum of the atomic masses of the.
Silver nitrate (CAS 7761888) Glentham Life Sciences
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in AgNO3: Molar Mass (g/mol) Ag (Silver) 1 × 107.8682 = 107.8682. N (Nitrogen) 1 × 14.0067 = 14.0067.
SOLVED Calculate the mass of silver nitrate (AgNO3) needed to make up 500 mL of a 1.00 mM
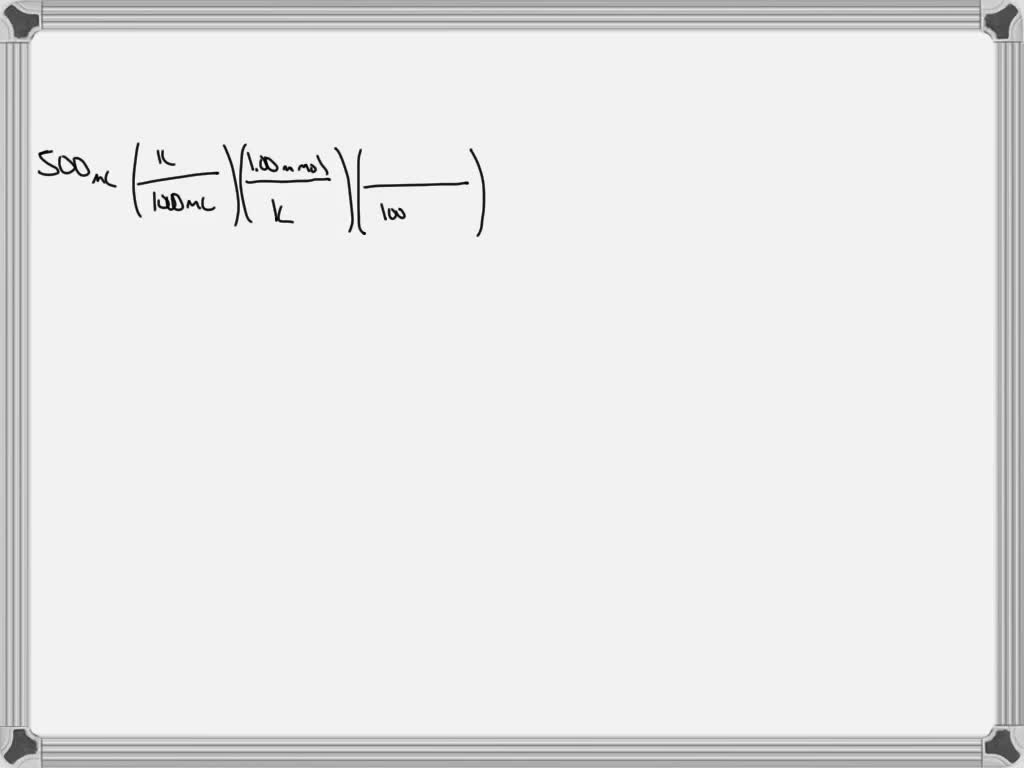
Molar Mass, Molecular Weight and Elemental Composition Calculator. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of AgNO3 (Silver nitrate) is 169.8731 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between AgNO3 weight and moles.
Silver Nitrate Symbol Periodic Table Awesome Home
169.8722. The molar mass of AgNO3 (Silver Nitrate) is: 169.8722 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set).
[Solved] Silver nitrate reacts with potassium iodide in aqueous solution to... Course Hero
Silver nitrate. Molecular Formula AgNO. Average mass 169.873 Da. Monoisotopic mass 168.892914 Da. ChemSpider ID 22878. - Charge.
[Solved] Calculate the molar concentration of 3.54g of silver nitrate,... Course Hero
Silver nitrate has a molar mass of 169.87 grams per mole. Silver nitrate does not have any color or odor. In a liquid state, silver nitrate has a density of 3.97 grams per cubic centimeter. In a solid-state, it is 4.35 grams per cubic centimeters. The melting point of silver nitrate is 482.8 Kelvin while the boiling point is 713 Kelvin.
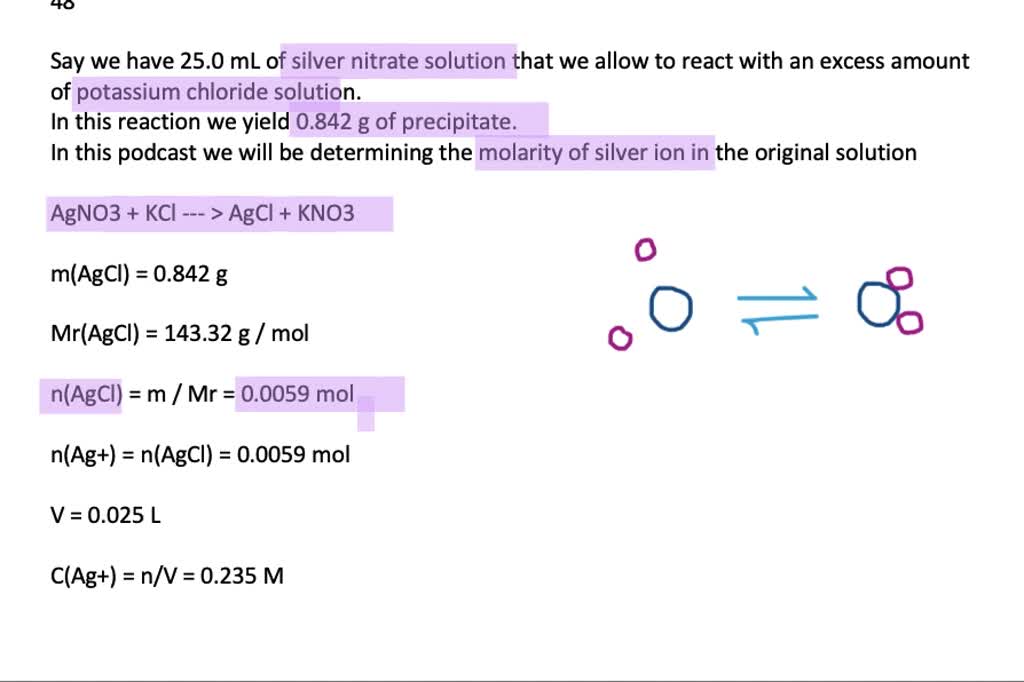
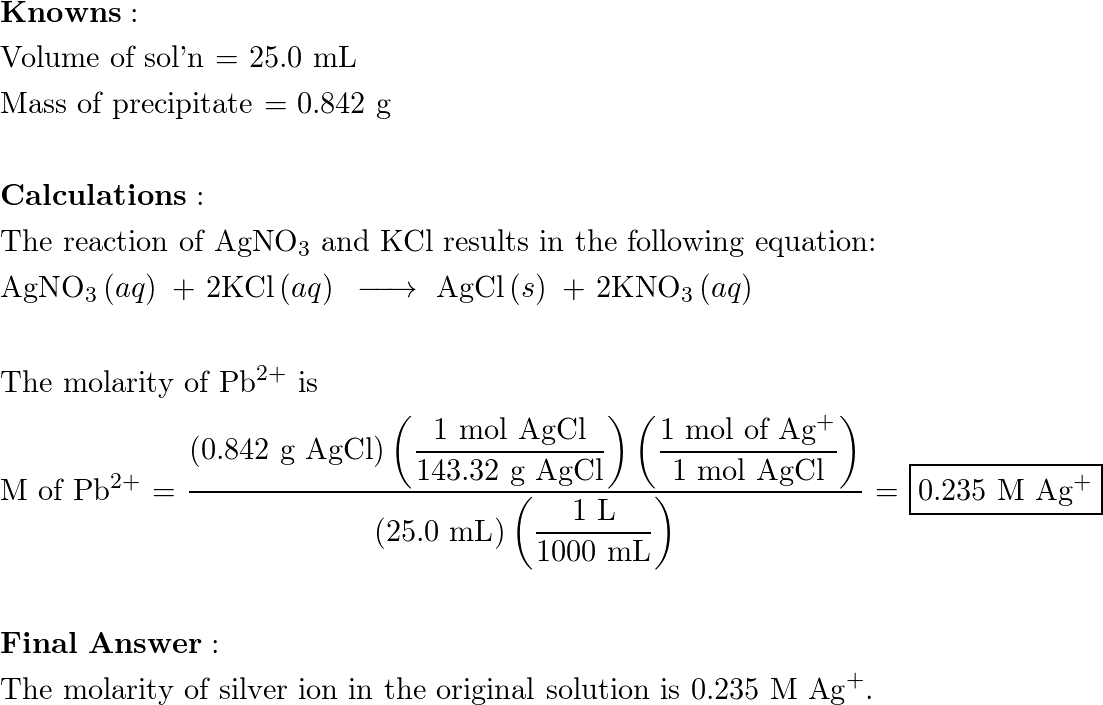
If 25.0 mL of silver nitrate solution reacts with excess pot Quizlet
Molar mass: 169.872 g·mol −1 Appearance colorless solid Odor: Odorless Density:. Std molar entropy (S. Silver nitrate is an inorganic compound with chemical formula AgNO 3. It is a versatile precursor to many other silver compounds, such as those used in photography.
Solved 1. How many moles of silver nitrate were added to the

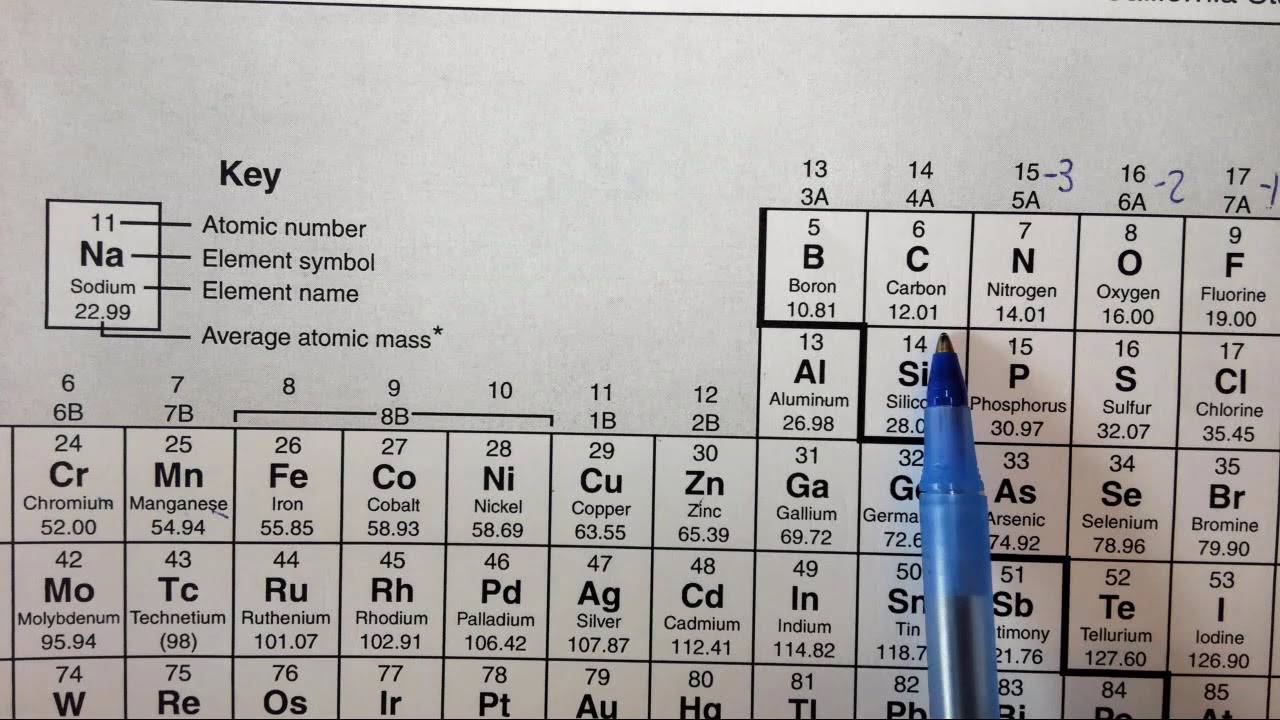
Use this periodic table for calculating molar mass for any chemical formula. Options for hiding the symbol or name of the elements provide a handy learning aid for memorizing the periodic table. iOS app is also available.. Silver 107.8682: 48 Cd Cadmium 112.414: 49 In Indium 114.818: 50 Sn Tin 118.710: 51 Sb Antimony 121.760: 52 Te Tellurium.
.